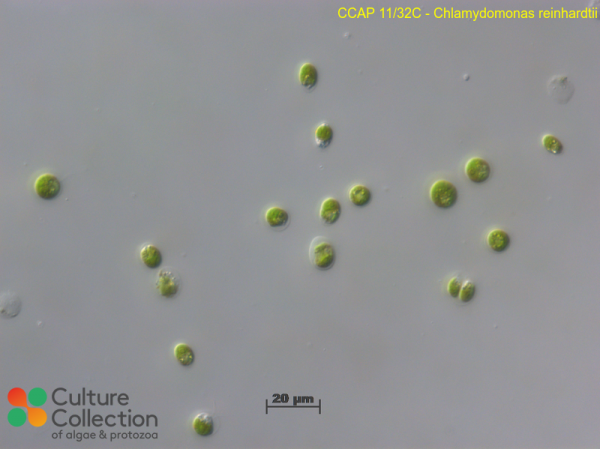
References [ 19 ]
Morris GJ, Coulson GE & Leeson EA (1985) Changes in the shape of mitochondria following osmotic stress to the unicellular green alga Chlamydomonas reinhardii. Journal of Cell Science 76: 145-153.
DOI: none
Reynoso GT & de Gamboa BA (1982) Salt tolerance in the freshwater algae Chlamydomonas reinhardtii: Effect of proline and taurine. Comparative Biochemistry and Physiology 73A(1): 95-99.
DOI: none
Pittman JK, Edmond C, Sunderland PA & Bray CM (2009) A cation-regulated and proton gradient-dependent cation transporter from Chlamydomonas reinhardtii has a role in calcium and sodium homeostasis. Journal of Biological Chemistry 284: 525-533.
Maberly SC & Spence DHN (1983) Photosynthetic inorganic carbon use by freshwater plants. Journal of Ecology 71: 705-724.
DOI: none
Clarke KJ & Leeson EA (1985) Plasmalemma structure in freezing tolerant unicellular algae. Protoplasma 129: 120-126.
Webster RE, Dean AP & Pittman JK (2011) Cadmium exposure and phosphorus limitation increases metal content in the freshwater alga Chlamydomonas reinhardtii. Environmental Science & Technology 45: 7489-7496.
Kebelmann K, Hornung A, Karsten U & Griffiths G (2013) Intermediate pyrolysis and product identification by TGA and Py-GC/MS of green microalgae and their extracted protein and lipid components. Biomass and Bioenergy 49: 38-48.
Morris GJ, Coulson G & Clarke A (1979) The cryopreservation of Chlamydomonas. Cryobiology 16: 401-410.
DOI: none
Driver T, Bajhaiya AK, Allwood JW, Goodacre R, Pittman JK & Dean AP (2015) Metabolic responses of eukaryotic microalgae to environmental stress limit the ability of FT-IR spectroscopy for species identification. Algal Research 11: 148-155.
Taylor NS, Merrifield R, Williams TD, Chipman JK, Lead JR & Viant MR (2015) Molecular toxicity of cerium oxide nanoparticles to the freshwater alga Chlamydomonas reinhardtii is associated with supra-environmental exposure concentrations. Nanotoxicology 10: 32-41.
Costa PM & Fadeel B (2016) Emerging systems biology approaches in nanotoxicology: Towards a mechanism-based understanding of nanomaterial hazard and risk. Toxicology and Applied Pharmacology 299: 101-111.
Larronde-Larretche M & Jin X (2017) Microalgal biomass dewatering using forward osmosis membrane: Influence of microalgae species and carbohydrates composition. Algal Research 23: 12-19.
Bekirogullari M, Fragkopoulos IS, Pittman JK & Theodoropoulos C (2017) Production of lipid-based fuels and chemicals from microalgae: An integrated experimental and model-based optimization study. Algal Research 23: 78-87.
Loera-Quezada MM, Leyva-González MA, Velázquez-Juárez G, Sanchez-Calderón L, Do Nascimento M, López-Arredondo D & Herrera-Estrella L (2016) A novel genetic engineering platform for the effective management of biological contaminants for the production of microalgae. Plant Biotechnology Journal 14: 2066-2076.
Bekirogullari M, Pittman JK & Theodoropoulos C (2018) Multi-factor kinetic modelling of microalgal biomass cultivation for optimised lipid production Bioresource Technology -: -.
Tassoni A, Awad N & Griffiths G (2018) Effect of ornithine decarboxylase and norspermidine in modulating cell division in the green alga Chlamydomonas reinhardtii Plant Physiology and Biochemistry 123: 125-131.
Dash A & Banerjee R (2018) In silico optimization of lipid yield utilizing mix-carbon sources for biodiesel production from Chlorella minutissima Energy Conversion and Management 164: 533-542.
Charles ED, Muhamadali H, Goodacre R & Pittman JK (2019) Biochemical signatures of acclimation by Chlamydomonas reinhardtii to different ionic stresses Algal Research 37: 83-91.
Figueroa-Torres GM, Mahmood WMAW, Pittman JK & Theodoropoulos C (2019) Microalgal Biomass as a Biorefinery Platform for Biobutanol and Biodiesel Production Biochemical Engineering Journal -: -.