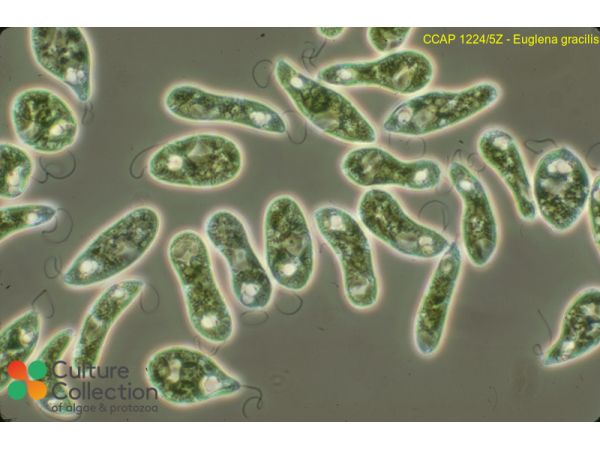
References [ 42 ]
Clegg MR, Maberley SC & Jones RI (2003) Behavioural responses of freshwater phytoplanktonic flagellates to a temperature gradient. European Journal of Phycology 38: 195-203.
Clegg MR, Maberley SC & Jones RI (2004) Dominance and compromise in freshwater phytoplanktonic flagellates: the interaction of behavioural preferences for conflicting environmental gradients. Functional Ecology 18: 371-380.
Clegg MR & Maberley SC (2003) The effect of photon irradiance on the behavioural ecology and potential niche separation of freshwater phytoplanktonic flagellates. Journal of Phycology 39: 650-662.
Clegg MR, Maberley SC & Jones RI (2003) Chemosensory behavioural response of freshwater phytoplanktonic flagellates. Plant, Cell and Environment 27: 123-135.
Clegg MR, Maberly SC & Jones RI (2007) Behavioural response as a predictor of seasonal depth distribution and vertical niche separation in freshwater phytoplanktonic flagellates. Limnology and Oceanography 52(1): 441-455.
DOI: none
Gachon CMM, Day JG, Campbell CN, Pröschold T, Saxon RJ & Küpper FC (2007) The Culture Collection of Algae and Protozoa (CCAP): A biological resource for protistan genomics Gene 406: 51-57.
Day JG (2004) Cryopreservation: fundamentals, mechanisms of damage on freezing/thawing and application in culture collections Nova Hedwigia 79: 191-205.
Fleck RA, Benson EE, Bremner DH & Day JG (2003) Studies of antioxidant protection in freeze-tolerant and freeze-sensitive microalgae: Applications in Cryopreservation protocol development. CryoLetters 24: 213-228.
DOI: none
Day JG, Fleck RA & Benson EE (2000) Cryopreservation-recalcitrance in microalgae: Novel approaches to identify and avoid cryo-injury. Journal of Applied Phycology 12: 369-377.
Fleck RA, Benson EE, Bremner DH & Day JG (2000) Studies of free radical-mediated cryoinjury in the unicellular green alga Euglena gracilis using a non-destructive hydroxyl radical assay: A new approach for developing protistan cryopreservation strategies. Free Radical Research 32: 157-170.
Johnstone C, Day JG, Staines H & Benson EE (2006) An in vitro oxidative stress test for determining pollutant tolerance in algae. Ecological Indicators 6: 770-779.
Fleck RA, Pickup RW, Day JG & Benson EE (2006) The use of flow-cytometry and cryomicroscopy to characterise cryopreservation-induced injuries in Euglena gracilis. Cryobiology 52: 261-268.
Freyssinent G, Eichholz RL & Buetow DE (1984) Kinetics of accumulation of Ribulose-1,5-biphosphate Carboxylase during greening in Euglena gracilis Plant Physiology 75(3): 850-857.
Whitehouse JW & Lewis BG (1973) The effect of diet and density on development, size and egg production in Cyclops abyssorum Sars, (Copepoda, Cyclopoida). Crustaceana 25(3): 225-236.
Zahalsky AC, Hutner SH, Keane M & Burger RM (1962) Bleaching Euglena gracilis with antihistamines and streptomycin-type antibiotics. Archiv für Microbiologie 42(1): 46-55.
Kahn JS (1984) Activation of coupling factor 1 from Euglena gracilis chloroplasts: Conditions for optimal activation and their possible physiological significance. Plant Physiology 75(2): 410-413.
Lonergan TA (1984) Regulation of cell shape in Euglena gracilis. Journal of Cell Science 71: 37-50.
DOI: none
Hyams JS (1982) The Euglena paraflagellar rod: Structure, relationship to other flagellar components and preliminary biochemical characterization. Journal of Cell Science 55: 199-210.
DOI: none
Axelsson-Olsson D, Olofsson J, Svensson L, Griekspoor P, Waldenström J, Ellström P & Olsen B (2010) Amoebae and algae can prolong the survival of Campylobacter species in co-culture. Experimental Parasitology 126: 59-64.
Javed Q & Merrett MJ (1986) Properties and immunochemical characterization of NADPH-specific glutamate dehydrogenase from Euglena gracilis. New Phytologist 104: 407-413.
DOI: none
Morris GJ & Canning CE (1978) The Cryopreservation of Euglena gracilis Journal of General Microbiology 108: 27-31.
Suzuki E, Tsuzuki M & Miyachi S (1986) Accumulation of inorganic carbon in the cells of Euglena gracilis Z grown photoautotrophically in ordinary air. Plant & Cell Physiology 27: 1205-1208.
DOI: none
Hashimoto H & Murakami S (1983) Effects of cycloheximide and chloramphenicol on chloroplast replication in synchronously dividing cultured cells of Euglena gracilis New Phytologist 94: 521-529.
DOI: none
Hwang S & Hudock GA (1971) Stability of Chlamydomonas reinhardi in liquid nitrogen storage. Journal of Phycology 7: 300-303.
Maberly SC, Courcelle C, Groben R & Gontero B (2010) Phylogenetically-based variation in the regulation of the Calvin cycle enzymes, phophoribulokinase and glyceraldehyde-3-phosphate dehydrogenase, in algae. Journal of Experimental Botany 61: 735-745.
Nowack ECM, Podola B & Melkonian M (2005) The 96-well twin-layer system: A novel approach in the cultivation of microalgae. Protist 156: 239-251.
Monfils AK, Triemer RE & Bellairs EF (2011) Characterization of paramylon morphological diversity in photosynthetic euglenoids (Euglenales, Euglenophyta). Phycologia 50: 156-169.
Bianchi VA, Castro JM, Rocchetta I, Nahabedian DE, Conforti V & Luquet CM (2015) Long-term feeding with Euglena gracilis cells modulates immune responses, oxidative balance and metabolic condition in Diplodon chilensis (Mollusca, Bivalvia, Hyriidae) exposed to living Escherichia coli. Fish & Shellfish Immunology 42: 367-378.
Taipale SJ, Peltomaa E, Hiltunen M, Jones RI, Hahn MW, Biasi C & Brett M (2015) Inferring phytoplankton, terrestrial plant and bacteria bulk δ13C values from compound specific analyses of lipids and fatty acids. PLoS ONE 10(7): e0133974.
Záhonová K, Füssy Z, Oborník M, Eliás M & Yurchenko V (2016) RuBisCO in non-photosynthetic alga Euglena longa: Divergent features, transcriptomic analysis and regulation of complex formation. PLoS ONE 11: e0158790.
Markunas CM & Triemer RE (2016) Evolutionary history of the enzymes involved in the Calvin-Benson cycle in Euglenids. Journal of Eukaryotic Microbiology 63: 326-339.
Aiyar P, Schaeme D, García-Altares M, Carrasco Flores D, Dathe H, Hertweck C, Sasso S & Mittag M (2017) Antagonistic bacteria disrupt calcium homeostasis and immobilize algal cells Nature Communications 8: 1756.
Tossavainen M, Chopra NK, Kostia S, Valkonen K, Sharma AK, Sharma S, Ojala A & Romantschuk M (2018) Conversion of biowaste leachate to valuable biomass and lipids in mixed cultures of Euglena gracilis and chlorophytes Algal Research 35: 76-84.
Shao Q, Hu L, Qin H, Liu Y, Tang X, Lei A & Wang J (2019) Metabolomic response of Euglena gracilis and its bleached mutant strain to light PLoS ONE 14(11): e0224926.
Jalilian N, Najafpour GD & Khajouei M (2020) Macro and Micro Algae in Pollution Control and Biofuel Production – A Review ChemBioEng Reviews 7: 18-33.
Peltomaa ET & Taipale S (2020) Osmotrophic glucose and leucine assimilation and its impact on EPA and DHA content in algae. PeerJ 8: e8363.
Tossavainen M, Ilyass U, Ollilainen V, Valkonen K, Ojala A & Romantschuk M (2019) Influence of long term nitrogen limitation on lipid, protein and pigment production of Euglena gracilis in photoheterotrophic cultures. PeerJ 7: e6624.
Mollo L, Petrucciani A & Norici A (2024) Selection of microalgae in artificial digestate: Strategies towards an effective phycoremediation Plant Physiology and Biochemistry 210: 108588.
Lu F, Wu G, Wu G, Zhang L, Want J, Liu Z & Wu M (2024) Enhancing wastewater remediation in microalga Euglena gracilis: The role of trivalent cerium (Ce3+) as a hormonal effect factor and its metabolic implications Process Safety and Environmental Protection 186: 1273-1285.
He Z, Wang J & Li Y (2024) Recent Advances in Microalgae-driven Carbon Capture, Utilization, and Storage: Strain Engineering through Adaptive Laboratory Evolution and Microbiome Optimization Green Carbon -: -.
Xin K, Cheng J, Guo R, Qian L, Wu Y & Yang W (2024) Nuclear mutagenesis and adaptive evolution improved photoautotrophic growth of Euglena gracilis with flue-gas CO2 fixation Bioresource Technology 397: 130497.
Rifna EJ, Rajauria G, Dwivedi M & Tiwari BK (2024) Circular economy approaches for the production of high-value polysaccharides from microalgal biomass grown on industrial fish processing wastewater: A review International Journal of Biological Macromolecules 254: 126887.