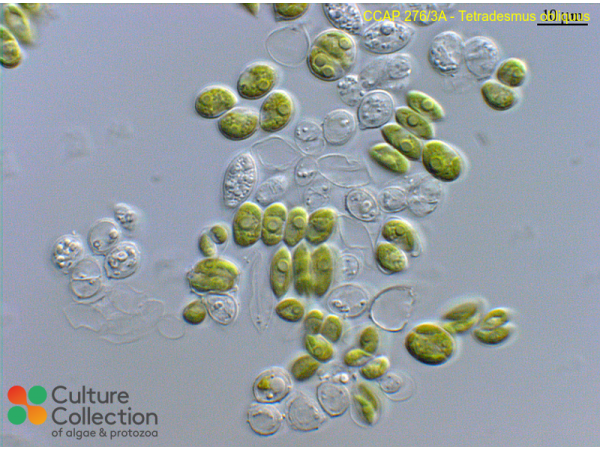
References [ 40 ]
Harding K, Müller J, Lorenz M, Timmerman H, Friedl T, Day JG & Benson EE (2008) Deployment of the encapsulation/dehydration protocol to cryopreserve microalgae held at Sammlung von Algenkulturen, Universität Göttingen Germany. CryoLetters 29: 15-20.
DOI: none
Cannell RJP, Owsianka AM & Walker JM (1988) Results of a large-scale screening programme to detect antibacterial activity from freshwater algae. European Journal of Phycology 23: 41-44.
Fallowfield HJ & Osbourne BA (1985) Growth and light absorbance of Cyanobacteria and Chlorophyceae with particular reference to Anabaena variabilis and Scenedesmus obliquus. British Phycological Journal 20(1): 27-41.
Hodaifa G, Martinez ME & Sánchez S (2008) Use of industrial wastewater from olive-oil extraction for biomass production of Scenedesmus obliquus. Bioresource Technology 99: 1111-1117.
Mulderij G, Mooij WM, Smolders AJP & Van Donk E (2005) Allelopathic inhibition of phytoplankton by exudates from Stratiotes aloides. Aquatic Botany 82: 284-296.
Hodaifa G, Martínez ME & Sánchez S (2009) Daily doses of light in relation to the growth of Scenedesmus obliquus in diluted three-phase olive mill wastewater. Journal of Chemical Technology & Biotechnology 84: 1550-1558.
Hodaifa G, Martínez ME, Órpez R & Sánchez S (2010) Influence of hydrodynamic stress in the growth of Scenedesmus obliquus using a culture medium based on olive-mill wastewater. Chemical Engineering and Processing: Process Intensification 49: 1161-1168.
Dionisio Pires LM, Ibelings BW, Brehm M & Van Donk E (2005) Comparing grazing on Lake Seaston by Dreissena and Daphnia: Lessons for biomanipulation. Microbial Ecology 50: 242-252.
DOI: none
Hodaifa G, Martínez ME, Órpez R & Sánchez S (2012) Inhibitory effects of industrial olive-oil mill wastewater on biomass production of Scenedesmus obliquus. Ecological Engineering 42: 30-34.
Matthijs HCP, Visser PM, Reeze B, Meeuse J, Slot PC, Wijn G, Talens R & Huisman J (2012) Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Research 46: 1460-1472.
Dionisio Pires LM, Sarpe D, Brehm M & Ibelings BW (2011) Potential synergistic effects of microcystins and bacterial lipopolysaccharides on life history traits of Daphnia galeata raised on low and high food levels. Aquatic Toxicology 104: 230-242.
Friedl T & O'Kelly CJ (2002) Phylogenetic relationships of green algae assigned to the genus Planophila (Chlorophyta): evidence from 18S rDNA sequence data and ultrastructure European Journal of Phycology 37: 373-384.
Hodaifa G, Sanchez S, Martinez ME & Orpez R (2013) Biomass production of Scenedesmus obliquus from mixtures of urban and olive-oil mill wastewaters used as culture medium. Applied Energy 104: 345-352.
Huss VAR, Frank C, Hartmann EC, Hirmer M, Kloboucek A, Seidel BM, Wenzeler P & Kessler E (1999) Biochemical taxonomy and molcular phylogeny of the genus Chlorella sensu lato (Chlorophyta). Journal of Phycology 35: 587-598.
DOI: none
Verschoor AM, van der Stap I, Helmsing NR, Lurling M & van Donk E (2004) Inducible colony formation within the Scenedesmaceae: Adaptive responses to infochemicals from two different herbivore taxa. Journal of Phycology 40: 808-814.
Hoham RW, Bonome TA, Martin CW & Leebens-Mack JH (2002) A combined 18S rDNA and rbcL phylogenetic analysis of Chloromonas and Chlamydomonas (Chlorophyceae, Volvocales) emphasizing snow and other cold-temperature habitats. Journal of Phycology 38: 1051-1064.
DOI: none
Krienitz L, Ustinova I, Friedl T & Huss VAR (2001) Traditional generic concepts versus 18S rRNA gene phylogeny in the green algal family Selenastraceae (Chlorophyceae, Chlorophyta). Journal of Phycology 37: 852-865.
DOI: none
Huss VAR, Frank C, Hartmann EC, Hirmer M, Kloboucek A, Seidel BM, Wenzeler P & Kessler E (1999) Biochemical taxonomy and molecular phylogeny of the genus Chlorella sensu lato (Chlorophyta) Journal of Phycology 35: 587-598.
DOI: none
Franchino M, Comino E, Bona F & Riggio VA (2013) Growth of three microalgae strains and nutrient removal from an agro-zootechnical digestate Chemosphere 92: 738-744.
Caisova L, Marin B & Melkonian M (2013) A consensus secondary structure of ITS2 in the Chlorophyta identified by phylogenetic reconstruction. Protist 164: 482-496.
Castrillo M, Lucas-Salas LM, Rodriguez-Gil C & Martinez D (2013) High pH-induced flocculation-sedimentation and effect of supernatant reuse on growth rate and lipid productivity of Scenedesmus obliquus and Chlorella vulgaris. Bioresource Technology 128: 324-329.
Lucas-Salas LM, Castrillo M & Martinez D (2013) Effects of dilution rate and water reuse on biomass and lipid production of Scenedesmus obliquus in a two-stage novel photobioreactor. Bioresource Technology 143: 344-352.
Gergs R, Steinberger N, Basen T, Martin-Creuzburg D (2014) Dietary supply with essential lipids affects growth and survival of the amphipod Gammarus roeselii. Limnologica 46: 109-115.
Massart A, Mirisola A, Lupant D, Thomas D & Hantson A (2014) Experimental characterization and numerical simulation of the hydrodynamics in an airlift photobioreactor for microalgae cultures. Algal Research 6: 210-217.
Ndikubwimana T, Zeng X, Liu Y, Chang JS & Lu Y (2014) Havesting of microalgae Desmodesmus sp. F51 by bioflocculation with bacterial bioflocculant. Algal Research 6B: 186-193.
Beuckels A, Smolders E & Muylaert K (2015) Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Research 77: 98-106.
Cunningham A (1990) A low-cost, portable flow cytometer specifically designed for phytoplankton analysis. Journal of Plankton Research 12: 149-160.
Dionisio Pires LM, Bontes BM, Van Donk E & Ibelings BW (2005) Grazing on colonial and filamentous, toxic and non-toxic cyanobacteria by the zebra mussel Dreissena polymorpha. Journal of Plankton Research 27: 331-339.
Whitton R, Le Mével A, Pidou M, Ometto F, Villa R & Jefferson B (2016) Influence of microalgal N and P composition on wastewater nutrient remediation. Water Research 91: 371-378.
Kapsetaki SE (2015) Predation and the evolution of multicellularity Thesis submitted for the degree of Master of Science (by Research) in Zoology; St. Hughs College, University of Oxford -: -.
DOI: none
Brans KI, Govaert L, Engelen JMT, Gianuca AT, Souffreau C & De Meester L (2016) Eco-evolutionary dynamics in urbanized landscapes: evolution, species sorting and the change in zooplankton body size along urbanization gradiants. Philosophical Transactions of the Royal Society of London. Series B 372: -.
Ji X, Verspagen JMH, Stomp M & Huisman J (2017) Competition between cyanobacteria and green algae at low versus elevated CO2: who will win, and why? Journal of Experimental Botany erx027: -.
Macke E, Callens M, De Meester L & Decaestecker E (2017) Host-genotype dependent gut microbiota drives zooplankton tolerance to toxic cyanobacteria Nature Communications 8: 1608.
Trivedi J, Atray N & Agrawal D (2018) Enhanced biomass production of Scenedesmus obliquus in a flat-panel photobioreactor, grown in photoautotrophic mode Biofuels -: -.
Rugnini L, Ellwood NTW, Costa G, Falsetti A, Congestri R & Bruno L (2019) Scaling-up of wastewater bioremediation by Tetradesmus obliquus, sequential bio-treatments of nutrients and metals Ecotoxicology and Environmental Safety 172: 59-64.
Qiu S, Wang L, Champagne P, Cao G, Chen Z, Wang S & Ge S (2019) Effects of crystalline nanocellulose on wastewater-cultivated microalgal separation and biomass composition Applied Energy 239: 207-217.
Murujew O, Whitton R, Kube M, Fan L, Roddick F, Jefferson B & Pidou M (2019) Recovery and reuse of alginate in an immobilized algae reactor Environmental Technology -: -.
Umamaheswari J & Shanthakumar S (2019) Phycoremediation of paddy-soaked wastewater by indigenous microalgae in open and closed culture system Journal of Environmental Management 243: 435-443.
Trivedi J, Atray N & Agrawal D (2020) Evaluating Cell Disruption Strategies for Aqueous Lipid Extraction from Oleaginous Scenedesmus obliquus at High Solid Loadings European Journal of Lipid Science and Technology 122: 1900328.
Liyanaarachchi VC, Premaratne M, Ariyadasa TU, Nimarshana PHV & Malik A (2021) Two-stage cultivation of microalgae for production of high-value compounds and biofuels: A review Algal Research 57: 102353.