References [ 28 ]
Gachon CMM, Day JG, Campbell CN, Pröschold T, Saxon RJ & Küpper FC (2007) The Culture Collection of Algae and Protozoa (CCAP): A biological resource for protistan genomics Gene 406: 51-57.
Whitton BA & MacArthur K (1967) The action of two toxic quinones on Anacystis nidulans. Archiv für Microbiologie 57: 147-154.
Weber HL & Boeck A (1968) Comparative studies on the regulation of DAHP synthetase activity in blue-green and green algae. Archiv für Microbiologie 61: 159-168.
Rippka R & Cohen-Bazire G (1983) The cyanobacteriales: A legitimate order based on the type strain Cyanobacterium stanieri Annals of Microbiology 134B: 21-36.
Wright SJL, Redhead K & Maudsley H (1981) Acanthamoeba castellanii, a predator of cyanobacteria. Journal of General Microbiology 125: 293-300.
Peschek GA (1983) Proton pump coupled to cytochrome c oxidase in the cyanobacterium Anacystis nidulans. Journal of Bacteriology 153: 539-542.
DOI: none
Renström-Kellner E & Bergman B (1990) Glycolate metabolism in cyanobacteria. IV. Uptake, growth and metabolic pathways. Physiologia Plantarum 78: 285-292.
Echlin P (1964) Intra-cytoplasmic membranous inclusions in the blue-green alga, Anacystis nidulans. Archiv für Microbiologie 49: 267-274.
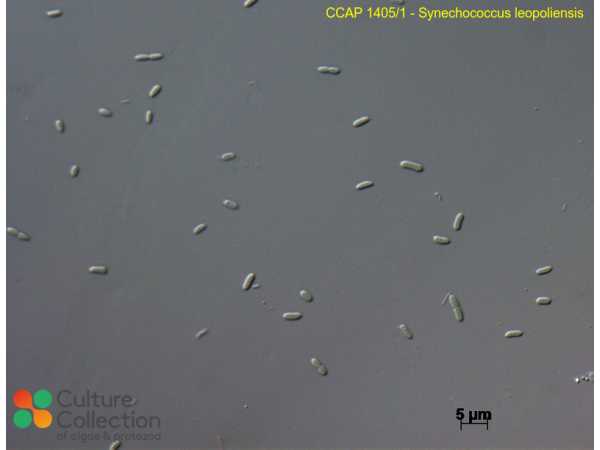
Noaman NH, Fattah A, Khaleafa M & Zaky SH (2004) Factors affecting antimicrobial activity of Synechococcus leopoliensis Microbiological Research 159: 395-402.
Miller B, Heuser T & Zimmer W (1999) A Synechococcus leopoliensis SAUG 1402-1 operon harboring the 1-deoxyxylulose 5-phosphate synthase gene and two additional open reading frames is functionally involved in the dimethylallyl diphosphate synthesis. FEBS Letters 460: 485-490.
DOI: none
Miller AG, Turpin DH & Canvin DT (1984) Growth and photosynthesis of the cyanobacterium Synechococcus leopoliensis in HCO3--limited chemostats. Plant Physiology 75: 1064-1070.
Mayo WP, Williams TG, Birch DG & Turpin DH (1986) Photosynthetic adaptation by Synechococcus leopoliensis in response to exogenous dissolved inorganic carbon. Plant Physiology 80: 1038-1040.
DOI: none
Gerbling K, Steup M & Latzko E (1985) Fructose-1,6-biphosphatase from Synechococcus leopoliensis. Substrate-dependent dimer - tetramer interconversion. European Journal of Biochemistry 147: 207-215.
Croisetière L, Rouillon R & Carpentier R (2001) A simple mediatorless amperometric method using the cyanobacterium Synechococcus leopoliensis for the detection of phytotoxic pollutants. Applied Microbiology and Biotechnology 56: 261-264.
Rigby CH, Craig SR & Budd K (1980) Phosphate uptake by Synechococcus leopoliensis (Cyanophyceae): Enhancement by calcium ion. Journal of Phycology 16: 389-393.
Martin-Nieto J, Flores E & Herrero A (1992) Biphasic kinetic behaviour of nitrate reductase from heterocystous, nitrogen-fixing cyanobacteria. Plant Physiology 100: 157-163.
DOI: none
Rippka R, Deruelles J, Waterbury JB, Herdman M & Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Journal of General Microbiology 111: 1-61.
Summons RE, Jahnke LL, Hope JM & Logan GA (1999) 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature 400: 554-557.
Cox PA, Banack SA, Murch SJ, Rasmussen U, Tien G, Bidigare RR, Metcalf JS, Morrison LF, Codd GA & Bergman B (2005) Diverse taxa of cyanobacteria produce ß-N-methylamino-L-alanine, a neurotoxic amino acid. PNAS 102: 5074-5078.
Yu J, Liberton M, Cliften PF, Head RD, Jacobs JM, Smith RD, Koppenaal DW, Brand JJ & Pakrasi HB (2015) Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Science Reports 5: 8132.
Hayashi S, Itoh K & Suyama K (2011) Growth of the cyanobacterium Synechococcus leopoliensis CCAP 1405/1 on agar media in the presence of heterotrophic bacteria. Microbes and Environments 26: 120-127.
Hayashi S, Itoh K & Suyama K (2015) Genes of Bacillus subtilis 168 that support growth of the cyanobacterium, Synechococcus leopoliensis CCAP 1405/1 on agar media. Microbial Ecology 70: 849-852.
Henson BJ, Hartman L, Watson LE & Barnum SR (2011) Evolution and variation of the nifD and hupL elements in the heterocystous cyanobacteria. International Journal of Systematic and Evolutionary Microbiology 61: 2938-2949.
Guo J, Selby K & Boxall A (2016) Comparing the sensitivity of chlorophytes, cyanobacteria and diatoms to major-use antibiotics. Environmental Toxicology and Chemistry 35: 2587-2596.
Chen X, Schreiber K, Appel J, Makowka A, Fähnrich B, Roettger M, Hajirezaei MR, Sönnichsen FD, Schönheit P, Martin WF & Gutekunst K (2016) The Entner-Doudoroff pathway is an overlooked glycolytic route in cyanobacteria and plants. PNAS 113: 5441-5446.
Golden SS (2018) The international journeys and aliases of Synechococcus elongatus New Zealand Journal of Botany -: -.
Le Page G, Gunnarsson L, Trznadel M, Wedgewood KCA, Baudrot V, Snape J & Tyler CR (2019) Variability in cyanobacteria sensitivity to antibiotics and implications for environmental risk assessment Science of the Total Environment 695: 133804.
Le Page G, Gunnarsson L, Snape J & Tyler CR (2019) Development and Application of a Microplate Assay for Toxicity Testing on Aquatic Cyanobacteria Environmental Toxicology and Chemistry 39: 705-720.