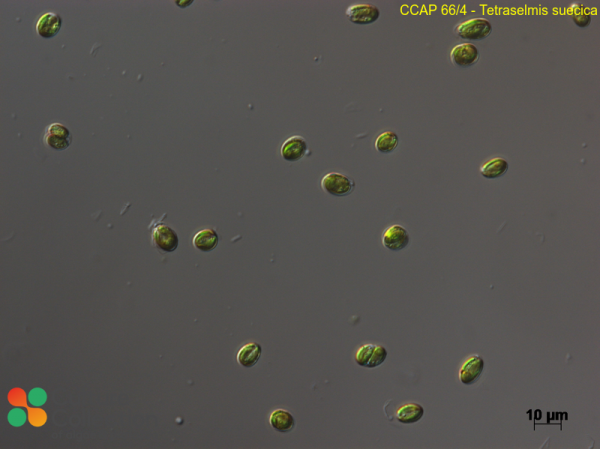
References [ 39 ]
Marques A, Dhont J, Sorgeloos P & Bossier P (2004) Evaluation of different yeast cell wall mutants and microalgae strains as feed for gnotobiotically grown brine shrimp Artemia franciscana. Journal of Experimental Marine Biology and Ecology. 312: 115-136.
Gooday GW (1970) A physiological comparison of the symbiotic algae Platymonas convolutae and its free-living relatives. Journal of the Marine Biological Association of the UK 50: 199-208.
DOI: none
Manton I & Parke M (1965) Observations on the fine structure of two species of Platymonas with special reference to flagellar scales and the mode of origin of the theca. Journal of the Marine Biological Association of the UK 45: 743-754.
DOI: none
Day JG (2004) Cryopreservation: fundamentals, mechanisms of damage on freezing/thawing and application in culture collections Nova Hedwigia 79: 191-205.
Day JG, Benson EE & Fleck RA (1999) In Vitro Culture and Conservation Of Microalgae: Applications For Environmental Research, Aquaculture & Biotechnology. In Vitro Cellular & Developmental Biology - Plant 35: 127-136.
Day JG, Watanabe MM & Turner MF (1998) Ex situ conservation of protistan and cyanobacterial biodiversity : CCAP - NIES collaboration 1991 - 1997. Phycological Research 46: 77-83.
DOI: none
Day JG, Watanabe MM, Morris GJ, Fleck RA & McLellan MR (1997) Long-term viability of preserved eukaryotic algae. Journal of Applied Phycology 9: 121-127.
Day JG & Fenwick C (1993) Cryopreservation of members of the genus Tetraselmis used in aquaculture. Aquaculture 118: 151-160.
Fenwick C & Day JG (1992) Cryopreservation of Tetraselmis suecica cultured under different nutrient regimes. Journal of Applied Phycology 4: 105-109.
Day JG (1998) Cryo-conservation of microalgae and cyanobacteria. CryoLetters S1: 7-14.
DOI: none
Sandaa R-A, Brunvold L, Magnesen T & Bergh Ø (2008) Monitoring the opportunistic bacteria Pseudoalteromonas sp. LT-13 in a great scallop, Pecten maximus hatchery. Aquaculture 276: 14-21.
Fuentes-Grünewald C, Garcés E, Rossi S & Camp J (2009) Use of the dinoflagellate Karlodinium veneficum as a sustainable source of biodiesel production. Journal of Industrial Microbiology and Biotechnology 36: 1215-1224.
Natrah FMI, Kenmegne MM, Wiyoto W, Sorgeloos P, Bossier P & Defoirdt T (2011) Effects of micro-algae commonly used in aquaculture on acyl-homoserine lactone quorum sensing. Aquaculture 317: 53-57.
Magnesen T & Jacobsen A (2012) Effect of water recirculation on seawater quality and production of scallop (Pecten maximus) larvae. Aquacultural Engineering 47: 1-6.
Redfearn P (1987) Larval shell development of the northern tuatua, Paphies subtriangulata (Bivalvia, Mesodesmatidae) New Zealand Journal of Marine and Freshwater Research 21: 65-70.
Montaini E, Chini Zittelli G, Tredici MR, Molina Grima E, Fernandez Sevilla JM & Sanchez Perez JA (1995) Long-term preservation of Tetraselmis suecica: Influence of storage on viability and fatty acid profile. Aquaculture 134: 81-90.
DOI: none
Lopez Barreiro D, Zamalloa C, Boon N, Vyverman W, Ronsse F, Brilman W & Prins W (2013) Influence of strain-specific parameters on hydrothermal liquefaction of microalgae Bioresource Technology 146: 463-471.
San Pedro A, Gonzalez-Lopez CV, Acien FG & Molina-Grima E (2013) Marine microalgae selection and culture conditions optimization for biodiesel production. Bioresource Technology 134: 353-361.
Wong DM & Franz AK (2013) A comparison of lipid storage in Phaeodactylum tricornutum and Tetraselmis suecica using laser scanning confocal microscopy. Journal of Microbiological Methods 95: 122-128.
Gonzalez-Araya R, Quillien V & Robert R (2013) The effects of eight single microalgal diets on sex-ratio and gonad development throughout European flat oyster (Ostrea edulis L.) conditioning. Aquaculture 400-401: 1-5.
Pronker AE, Peene F, Donner S, Wijnhoven S, Geijsen P, Bossier P & Nevejan NM (2013) Hatchery cultivation of the common cockle (Cerastoderma edule L.): From conditioning to grow-out. Aquaculture Research -: 1-11.
Goiris K, Muylaert K, Voorspoels S, Noten B, De Paepe D, Baart GJE & De Cooman L (2014) Detection of flavenoids in microalgae from different evolutionary lineages. Journal of Phycology 50: 483-492.
D'Alvise PW, Lillebo S, Prol-Garcia MJ, Wergeland HI, Nielsen KF, Bergh O & Gram L (2012) Phaeobacter gallaeciensis reduces Vibrio anguillarum in cultures of microalgae and rotifers, and prevents vibriosis in cod larvae. PLoS ONE 7(8): e43996.
Prol Garcia MJ, D'Alvise PW & Gram L (2013) Disruption of cell-to-cell signaling does not abolish the antagonism of Phaeobacter gallaeciensis toward the fish pathogen Vibrio anguillarum in algal systems. Applied and Environmental Microbiology 79: 5414-5417.
Goiris K, Van Colen W, Wilches I, León-Tamariz F, De Cooman L & Muylaert K (2015) Impact of nutrient stress of antioxidant production in three species of microalgae. Algal Research 7: 51-57.
Wong DM, Nguyen TTN & Franz AK (2014) Ethylenediaminetetraacetic acid (EDTA) enhances intracellular lipid staining with Nile red in microalgae Tetraselmis suecica. Algal Research 5: 158-163.
Giordano M, Palmucci M & Raven JA (2015) Growth rate hypothesis and efficiency of protein synthesis under different sulfate concentrations in two green algae. Plant, Cell and Environment 38: 2313-2317.
Porsby CH & Gram L (2016) Phaeobacter inhibens as biocontrol agent against Vibrio vulnificus in oyster models. Food Microbiology 57: 63-70.
Prioretti L, Lebrun R, Gontero B & Giordano M (2016) Redox regulation of ATP sulfurylase in microalgae Biochemical and Biophysical Research Communications 478: 1555-1562.
Lama S, Muylaert K, Karki TB, Foubert I, Henderson RK & Vandamme D (2016) Flocculation properties of several microalgae and a cyanobacterium species during ferric chloride, chitosan and alkaline flocculation. Bioresource Technology 220: 464-470.
Prioretti L & Giordano M (2016) Direct and indirect influence of sulfur availability on phytoplankton evolutionary trajectories. Journal of Phycology 52: 1094-1102.
Postma PR, Suarez-Garcia E, Safi C, Yonathan K, Olivieri G, Barbosa MJ, Wijffels RH & Eppink MHM (2017) Energy efficient bead milling of microalgae: Effect of bead size on disintegration and release of proteins and carbohydrates Bioresource Technology 224: 670-679.
Daneshvar E, Zarrinmehr MJ, Hashtjin AM, Farhadian O & Bhatnagar A (2018) Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption Bioresource Technology 268: 523-530.
González-Araya R & Robert R (2018) Larval development and fatty acid composition of Ostrea edulis (L.) fed four different single diets from conditioning to pre‐settlement Aquaculture Research 49: 1768-1781.
Fitzer SC, Plancq J, Floyd CJ, Kemp FM & Toney JL (2019) Increased pCO2 changes the lipid production in important aquacultural feedstock algae Isochrysis galbana, but not in Tetraselmis suecica Aquaculture and Fisheries 4: 142-148.
Diéguez AL, Balboa S, Magnesen T, Jacobsen A, Lema A & Romalde JL (2019) Comparative study of the culturable microbiota present in two different rearing systems, flow‐through system (FTS) and recirculation system (RAS), in a great scallop hatchery Aquaculture Research 51: 542-556.
Gwo J-C, Twan W-H & Lin S-C (2023) Cryopreservation of six marine microalgae used in Taiwanese aquaculture. Journal of the World Aquaculture Society 54(5): 1235-1246.
Roager L, Kempen PJ, Bentzon-Tilia M, Sonnenschein EC & Gram LO (2024) Impact of host species on assembly, composition, and functional profiles of phycosphere microbiomes. mSystems 0: e00583-24.
Kalamaras G, Antonopoulou M, Beobide AS, Triantafyllidis V & Dailianis S (2024) Disposable face masks into aquatic media: Chemical and biological testing of the released compounds during the leaching process Environmental Pollution 363: 125290.