References [ 29 ]
Day JG, Benson EE & Fleck RA (1999) In Vitro Culture and Conservation Of Microalgae: Applications For Environmental Research, Aquaculture & Biotechnology. In Vitro Cellular & Developmental Biology - Plant 35: 127-136.
Liu H, Kelly MS, Campbell DA, Dong SL, Zhu JX & Wang SF (2007) Exposure to domoic acid affects larval development of king scallop Pecten maximus (Linnaeus, 1758) Aquatic Toxicology 81: 152-158.
Christophersen G, Torkildsen L & van der Meeren T (2006) Effect of increased water recirculation rate on algal supply and post-larval performance of scallop (Pecten maximus) reared in a partial open and continuous feeding system. Aquacultural Engineering 35: 271-282.
Sandaa R-A, Brunvold L, Magnesen T & Bergh Ø (2008) Monitoring the opportunistic bacteria Pseudoalteromonas sp. LT-13 in a great scallop, Pecten maximus hatchery. Aquaculture 276: 14-21.
Nyström B, Björnsäter B & Blanck H (1999) Effects of sulfonylurea herbicides on non-target aquatic micro-organisms. Growth inhibition of micro-algae and short-term inhibition of adenine and thymidine incorporation in periphyton communities. Aquatic Toxicology 47: 9-22.
DOI: none
Natrah FMI, Kenmegne MM, Wiyoto W, Sorgeloos P, Bossier P & Defoirdt T (2011) Effects of micro-algae commonly used in aquaculture on acyl-homoserine lactone quorum sensing. Aquaculture 317: 53-57.
Magnesen T & Jacobsen A (2012) Effect of water recirculation on seawater quality and production of scallop (Pecten maximus) larvae. Aquacultural Engineering 47: 1-6.
Jacobsen A, Grahl-Nielsen O & Magnesen T (2012) Effects of reduced diameter of bag cultures on content of essential fatty acids and cell density in a continuous algal production system. Journal of Applied Phycology 24: 109-116.
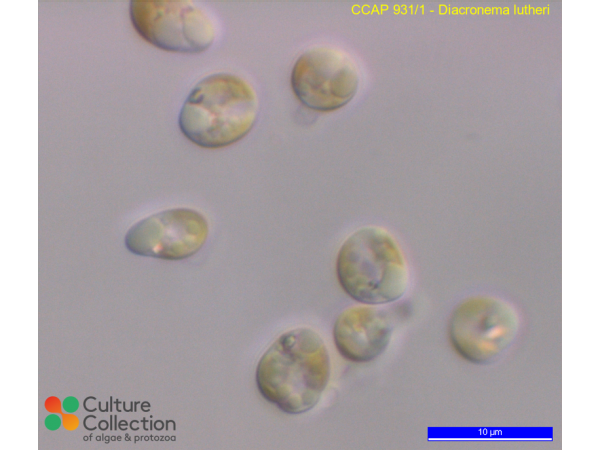
Bendif EM, Probert I, Hervé A, Billard C, Goux D, Lelong C, Cadoret J & Véron B (2011) Integrative taxonomy of the Pavlovophyceae (Haptophyta): A reassessment. Protist 162: 738-761.
Redfearn P (1987) Larval shell development of the northern tuatua, Paphies subtriangulata (Bivalvia, Mesodesmatidae) New Zealand Journal of Marine and Freshwater Research 21: 65-70.
Volkman JK, Jeffrey SW, Nichols PD, Rogers GI & Garland CD (1989) Fatty acid and lipid composition of 10 species of microalgae used in mariculture Journal of Experimental Marine Biology and Ecology 128: 219-240.
DOI: none
Gonzalez-Araya R, Lebrun L, Quere C & Robert R (2012) The selection of an ideal diet for Ostrea edulis (L.) broodstock conditioning (part B). Aquaculture 362-363: 55-66.
Rico-Villa B, Le Coz JR, Mingant C & Robert R (2006) Influence of phytoplankton diet mixtures on microalgae consumption, larval development and settlement of the Pacific oyster Crassostrea gigas (Thunberg) Aquaculture 256: 377-388.
Davidson K, Roberts EC, Wilson AM & Mitchell E (2005) The role of prey nutritional status in governing protozoan nitrogen regeneration efficiency. Protist 156: 45-62.
Leonardos N & Lucas IAN (2000) The use of larval fatty acids as an index of growth in Mytilus edulis L. larvae. Aquaculture 184: 155-166.
DOI: none
Leonardos N & Lucas IAN (2000) The nutritional value of algae grown under different culture conditions for Mytilus edulis L. larvae. Aquaculture 182: 301-315.
DOI: none
Madariaga I de & Joint I (1992) A comparative study of phytoplankton physiological indicators. Journal of Experimental Marine Biology and Ecology 158: 149-165.
DOI: none
Ehara M, Watanabe KI, Kawai H, Inagaki Y, Hayashi-Ishimaru Y & Ohama T (1998) Distribution of the mitochondrial deviant genetic code AUA for methionine in heterokont algae. Journal of Phycology 34: 1005-1008.
DOI: none
Gonzalez-Araya R, Quillien V & Robert R (2013) The effects of eight single microalgal diets on sex-ratio and gonad development throughout European flat oyster (Ostrea edulis L.) conditioning. Aquaculture 400-401: 1-5.
Goiris K, Muylaert K, Voorspoels S, Noten B, De Paepe D, Baart GJE & De Cooman L (2014) Detection of flavenoids in microalgae from different evolutionary lineages. Journal of Phycology 50: 483-492.
Yoon HS, Hackett JD & Bhattacharya D (2002) A single origin of the peridinin- and fucoxanthin- containing plastids in dinoflagellates through tertiary endosymbiosis. PNAS 99: 11724-11729.
Yoon HS, Hackett JD, Pinto G & Bhattacharya D (2002) The single, ancient origin of chromist plastids PNAS 99: 15507-15512.
Lama S, Muylaert K, Karki TB, Foubert I, Henderson RK & Vandamme D (2016) Flocculation properties of several microalgae and a cyanobacterium species during ferric chloride, chitosan and alkaline flocculation. Bioresource Technology 220: 464-470.
Ridley CJA, Parker BM, Norman L, Schlarb-Ridley B, Dennis R, Jamieson AE, Clark D, Skill SC, Smith AG & Davey MP (2018) Growth of microalgae using nitrate-rich brine wash from the water industry Algal Research 33: 91-98.
Bernaerts TMM, Gheysen L, Kyomugasho C, Kermani ZJ, Vandionant S, Foubert I, Hendrickx ME & Van Loey AM (2018) Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides Algal Research 32: 150-161.
Diéguez AL, Balboa S, Magnesen T, Jacobsen A, Lema A & Romalde JL (2019) Comparative study of the culturable microbiota present in two different rearing systems, flow‐through system (FTS) and recirculation system (RAS), in a great scallop hatchery Aquaculture Research 51: 542-556.
Penhaul Smith JK, Beveridge C, Laudicella VA, Hughes AD, McEvoy L & Day JG (2021) Utilising mixotrophically cultured "designer algae" as blue mussel larval feed. Aquaculture International -: -.
McLeod AR, Brand T, Campbell CN, Davidson K & Hatton A (2021) Ultraviolet radiation drives emission of climate-relevant gases from marine phytoplankton. Journal of Geophysical Research: Biogeosciences 126: 9.
Lewis AM (2023) An evaluation of the risks to food safety and shellfish farming in Great Britain, posed by marine biotoxins from, current and future emerging, marine microalgal species. A Thesis presented for the degree of Doctor of Philosophy at the University of Westminster -: -.